Many student of biology are not so familiar with the mathematical principles of spectrophotometer based methods of analysis. Many of them might be feeling that biology can be studied without much use of mathematics, which is, unfortunately not true. Here, in this article, we will seek some simplification of analytical part of spectrophotometric method which is used for the estimation of different biomolecules on a daily basis by industries and research laboratories.
Measurements is not a new thing to us, for example, we measure the quantity of the grocery items routinely and this is possible because all the grocery items posses important basic property i.e. mass. Having a quantifiable property, like mass of a substance, is essential for any kind of measurement. Therefore, different methods of measurements are being used for the quantification of different substances.
However, in scientific experiments, it is not as easy as it may appear because most of the molecules have microscopic structures and minute masses. This becomes a major hurdle when we are working with biomolecules like protein, DNA etc. Practically, it is very much impossible to directly measure the mass of a dissolved DNA in solution and prepare solutions of desired concentrations required for different experimental procedures, like polymerase chain reaction.
Research in molecular biology has been significantly accelerated by the innovative methodologies of estimation of biomolecules such as spectrophotometer. Therefore, it is very much essential to know the quantifiable property of the molecule which can give us numerical value of the amount of the molecules present in the solution and help us in its estimation.
If we look at the molecular structure of some biomolecules like DNA, amino acids and peptide bonds of the protein molecule which contain special chemical structures called as chromophores. Chromophores are sub molecular structures with a special ability to interact with electro-magnetic radiation and absorb it.
Following chromophores are used for protein estimation by spectrophotometer
- Amino acid R groups
- Peptide bonds between two amino acids
- Modified structures such as prosthetic groups (Haem)
Along with the chromophores, presence of conjugated double bonds (alternate single and double bonds in a molecule) also helps to a greater extent in the measurements. All these quantifiable properties of biomolecules makes it possible to estimate their concentration in the solution by spectrophotometer.
As per the Beer–Lambert law, the amount of light absorbed by the chromophores in the molecule is linearly proportional to the number of chromophores. One molecule can absorb more than one type of wavelengths from the UV-visible spectrum due to the fact that a single molecule may contain different conjugated bonds as well as different chromophores (e.g. carotenoids). However, only certain wavelengths will be absorbed maximally and this wavelength is termed as absorption maxima.
Following are important biomolecules and their absorption maxima
| Substance | Method | Wavelength (in nm) |
| DNA | Direct | 260 |
| Protein | Direct | Tyr, Trp: 278, Peptide bond 190 |
| Carotenoid | Direct | 420, 450, 480 |
| Porphyrins | Direct | 400 |
Absorption maxima for each compound to be quantified needs to be determined beforehand in order to accurately measure the concentration of the molecule. This is because a compound can generate a strong response at low concentration increasing the sensitivity of the assay.
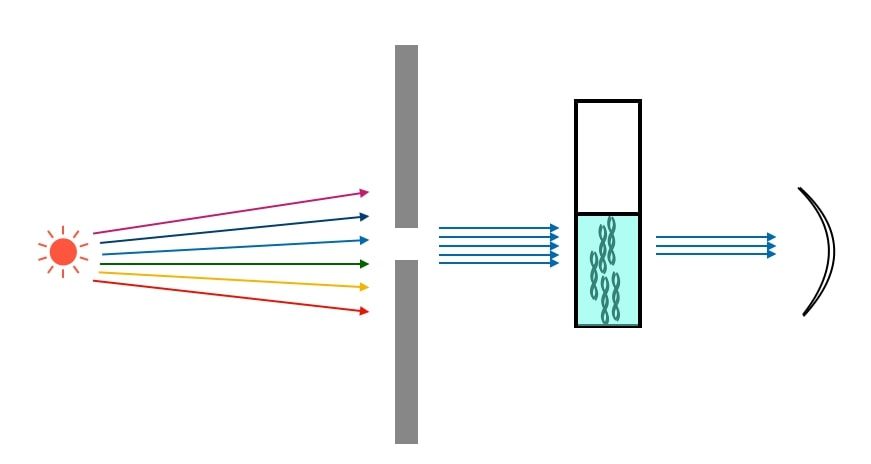
The sophisticated construction of the spectrophotometer enables us to measure the concentration of the compound (provided a standard curve analysis is performed beforehand) in the solution by measuring the amount of the fraction of the light transmitted when passed through the solution.
It is said that the concentration of the compound is directly proportional to the fraction of the light that is absorbed by the chromophores and conjugated double bonds of the molecules in the solution. However, the instrument can only measure the fraction of light that is transmitted through the solution, which means that, it does not measure the absorbance directly. This transmittance is measured and then used to determine the absorbance value and subsequently to determine the concentration of the compound by using standard response curve of the corresponding compound.
The quantification by measuring the absorption and their related mathematical expressions will be discussed in the next article in detail.
Read about PCR – Principle, definition, history, components and applications .

