Most commonly taught example of a reaction is,
A+B =>C
In the chemical terms it is explained to us and we think that now we have learnt what chemical reaction means. By believing this, we often underestimate the true nature of the reaction mechanism. It is not emphasised how crucial it is to know what are the implications of the reactions and how closely we are associated to different chemical ractions.
Chemical reactions are much more than just interactions of two molecular species. Millions of chemical reactions together form the foundation of life itself. Science tells us that the successful reaction is a chance event and depends on multiple factors. So we can think of life made up of millions of reactions as an event one of its kind.

Chemical reactions are a complex events but essential for life. Thus we need to understand the underlying facts about the reactions.
So when reactants A and B come in contact with each other, they don’t always react and form the product C. There are multiple environmental as well as thermodynamic factors that influence the product formation, in chemistry. Thermodynamic factors include the energy of the reactant and environmental factors include the presence of catalysts or inhibitor, temperature, pressure concentration of reactants, etc. that may alter the rate of product formation. Reaction won’t take place if any of the conditions is not fulfilled which is the case on most of the occasions.
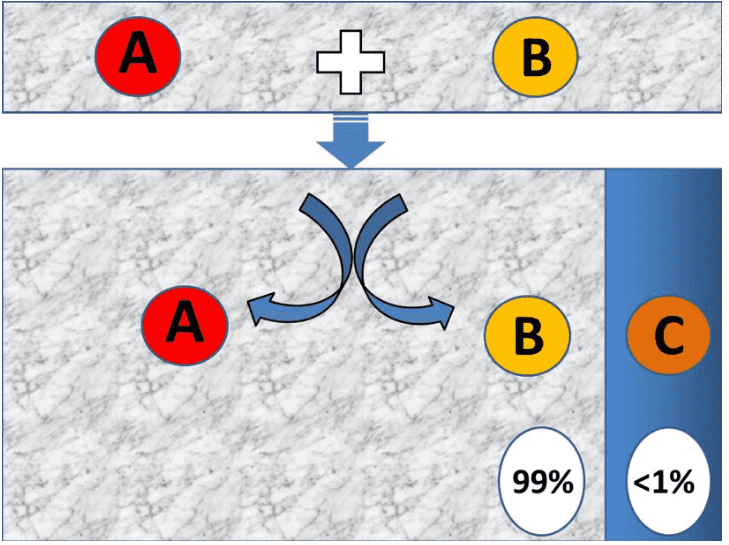
All texts explain how the reaction happens but very few mention when they don’t take place and why do they take place. In two parts we will discuss why the reaction takes place and how does it take place. In this article, we will see, what happens with reacting molecules i.e. reactants so they interact and form the product. And what makes them lose their original identity and transform them into altogether different forms.
The reaction is any response as a result of some action. It can be a smile or laughter, it can be anger and sometimes it is a slap too. But these reactions are of human sentiments, here; we want to explore the functionality of chemistry in biology. In scientific studies, reactions are physical and chemical. There are no biological reactions as such because biology is complex chemistry. The area of study is commonly known as biochemistry.
Physical reactions are attributed to changes in the physical properties of an object. Physical properties that can change are temperature, pressure, shape, and state of matter, etc. The dent on the car, roasting roti, making dough, water vaporization are some of the examples of physical reaction. In chemical sciences the process of reaction is fundamental and the biological system is a culmination of the process.
In every system energy makes things happen. The system is comprised of matter and thus more the energy more the instability in the matter is. Naturally, unstable objects have the tendency to give off the energy to attain stability. This indeed forms the basis for the process of reaction. Multiple physical, chemical, and nuclear reactions are well-known examples. So the process can be called a reaction that transforms the participants from the state of high energy to the state of lowest energy possible in the given environment. The reason for this is the state of matter with the lowest energy is favored by nature.
Same way if enough amount of energy is provided to the stable material, it becomes unstable. So for stability material has to lose the energy which it has gained earlier. The common mechanisms by which the energy is released are the emission of light, heat, radioactivity, forming new chemical bonds, etc. Imagine cooling of the soil after sundown. The cup of a hot coffee get colder by losing heat to the air. We will take a look at the examples of physical, chemical, and nuclear reactions.
Physical reaction :
When an iron bar heated (supply of energy from outside) it becomes hot (unstable state) with more heating it gets hotter. Once it is unable to accommodate more energy it starts radiating the energy (releasing energy to surrounding) in the form of electromagnetic radiation that is light and infrared rays. Again if more heat is given it starts melting and turns into a liquid with more heating. That’s when the iron casting is performed and again it is allowed to cool down (release of energy in order to stabilize). The casted iron bar might have gotten other shape but it’s still an iron. And that’s why it is called physical reactions.

Chemical reaction :
The consumption of food by an average human is a few tons in his lifetime. But the average weight of a person is around 50-70 kg. So where did the other part of the food go? This is an obvious question one might have. The food that we eat is a bunch of compound synthesized mainly by chemical reactions in plants. Thus the energy trapped in the chemical bonds of food is used to build our body.
In photosynthesis, the energy trapped from the sunlight is stored in the form of reduced carbon dioxide. The carbon dioxide is now called as carbohydrate, rich in energy. Step by step the energy from the carbohydrate, sugars in general terms, is drawn to carry out the daily activities after the consumption of the food. And at the end same carbon dioxide with the lowest energy is released into the environment through exhalation. The same goes for the burning of wood. It burns automatically because it is already loaded with energy and the wood wants to lose that energy. Burning helps the wood to lose energy faster.

Nuclear reaction :
The reactions are called nuclear reactions because the energy transactions are carried out at a level of subatomic particles. The nucleus of such elements is highly unstable because of the excess neutron or protons or both. Such elements emit energy in multiple forms. Heavy elements like Actinium emit alpha particles in order to reduce the excess nuclear particle. Some emit neutron or some elements just emit electromagnetic radiations like gamma rays. All these are the way for radioactive elements to achieve stability. Sometimes the nuclei of light elements fuse in a very high energy environment giving off an enormous amount of energy in the form of electromagnetic radiation, we call it sunlight. The heavy nucleus formed is more stable.
Thus the system is alive with energy and making this world so exuberant. People who are happy all the time and enthusiastic we usually articulate them as an energetic person. So be spirited and the change will be waiting for you. Living timid life is not going to lead anywhere.
Read about Know Mathematics To Know Biology Better.
(More information about chemistry of biological reaction)


