In the previous article we discussed about the synthesis of food by trapping the energy from the sunlight into the atmospheric carbon through the process of photosynthesis.. Here we will learn further journey of the carbon through glucose oxidation that provides us the usable energy. Glucose molecules contain 6 carbons reduced with high energy electrons. But not all the biological reactions can’t use the energy directly form the glucose.
It is necessary to transfer the energy from the reduced molecules into some easily redeemable chemical bonds by the oxidation of energy rich molecules. Bond formation in biological reactions use energy that is released during the oxidation. But in a minute, millions of reactions take place, how cells manage to provide energy to all the reactions at same time. Also energy providing molecule should only provide energy without disturbing the homeostasis. Glucose oxidation certainly can not meet an energy demand of all biological processes because glucose is at centre of many important metabolic pathways.
One more important thing is that, the energy needs to be stored for the immediate supply to the reactions in the absence of glucose. ATP molecules fulfils the important characters of ideal energy transferring molecule and has evolved to provide the energy to all the biological reactions. There are multiple reasons why ATP is called as energy currency. How the energy of glucose is moved to bond energy in the ATP molecule?
Glycolysis / Glucose oxidation
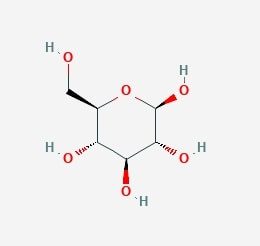
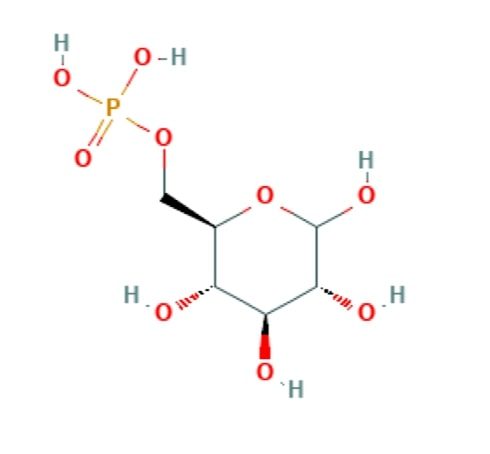
Glucose is six carbon molecule and is oxidised in stepwise manner. The process of breaking down of glucose or glucose oxidation is called glycolysis. Glucose is phosphorylated to activate and immobilise the glucose in the cytoplasm before it is oxidised as the cell membrane is impermeable to the glucose-6-phosphate. The glycolysis pathway has been evolved to extract maximum amount of energy stored in glucose molecule. Before the ATP yield from glucose is harvested two ATP molecules need to be invested to prepare glucose for optimum oxidation. One ATP is required for the phosphorylation of the glucose forming glucose-6-phosphate this molecule is then isomerised to fructose-6-phosphate.
Fructose-6-phosphate is again phosphorylated at first carbon forming fructose 1,6- bisphosphate. This investment of the of the ATP molecules is essential at this stage because fructose 1,6- bisphosphate can be broken down to two molecules of three carbons. These molecules can be efficiently oxidised and give the good payoff of the investment. These molecules are dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. Final products of glycolysis are 2 molecules of each NADH, pyruvate and ATP.
TCA cycle: a next phase of glucose oxidation
The next step is TCA cycle, where pyruvate is further processed into mitochondria of the cells in case of the eukaryote cells and in cytoplasm in case of the bacteria. In tricarboxylic acid cycle high energy electrons from a pyruvate are transferred to NAD+ forming NADH releasing CO2 molecules. This is the same CO2 that we exhale every minute after being processed through TCA cycle. The next and last step of the energy extraction process is an electron transport chain which is present in the inner mitochondrial membrane in eukaryotes and in the plasma membrane of the bacteria.
Electron transport chain
All NADH molecules formed from glycolysis and the tricarboxylic acid cycle donate the electrons to the electron acceptors in electron transport chain. Electron acceptors transfer the electron to the next molecule in the electron transport chain and during this transfer large amount of energy is released. This energy is used in the formation of the electrochemical gradient across the membrane as the formation the transmembrane gradient is a endergonic process . This transmembrane gradient facilitate the synthesis of ATP molecule.
The chemiosmotic mechanism utilises the potential difference formed across the membrane for the synthesis of the ATP molecules. This is how the immobilised energy from the sun in plants is further channelized towards ATP synthesis and these ATPs fuel the biological reactions.
But where does the electron go at the end? This electron is ultimately transferred to the oxygen that we inhale and reduce the oxygen forming water (H2O) molecule. It is fascinating that plants use H2O and CO2 to immobilise the energy and we give off the H2O and CO2 after using that energy.
Do solve the following Crossword & Test your Knowledge about The Carbs!