Before reading further please go through these concepts
Oxidation Reduction, Spontaneous Reactions, pKa, uphill and downhill process, reduction potential, NADH, ATP, Coupled reactions in biology, Glucose oxidation and Photosynthesis, Alpha Carbon.
Electron, Sun and Energy cycle
Many biology students know electron as a subatomic particle that continuously revolves around atomic nucleus and occasionally participating in chemical reactions. In the previous article we have seen how electron influences the molecular behaviour. Electronic transactions are of great significance in biology so as its ability to absorb, carry and emission of energy. In this article we shall see role and significance of electron from the biological perspective.
It is known to everyone that gain of electron is the reduction while losing it, is an oxidation. What goes underestimated is the energy of electron posses at that moment of electron transfer. This is measured in terms of the reduction potential of the compound bearing the high energy electrons. Reduction potential of any compound determines the affinity of the compound to accept the electron.
When we leave a ball from 8th floor of the building, it falls on the ground within seconds. This process is called as a spontaneous process because a ball did not need anyone to carry it downstairs. But when we want to get the ball on 8th floor, someone will have to go and carry it. As per the laws of thermodynamics any spontaneous process can run in one direction i.e. downhill, which does not require any external source of energy for the execution of the process as it happened when ball was falling from 8th floor of the building.
The process is called, uphill process when it needs external input of energy for the execution of the process as like getting a ball to the 8th floor of the building from the ground floor. Compounds with only certain reduction potential can transfer the electron to appropriate compounds and not the every other compound.
It means that compound bearing a low energy electron (the one going downhill) can not transfer the same to the compound which otherwise carries the high energy electron. And that is why it is stepwise transfer of the electron and energy. Thus NOT every compound can transfer electron to any other compound to reduce it. Most of the biological reactions are carried out in both way i.e. downhill and uphill.
When electrons are high in energy i.e. when they are at the excited state they tend to loose the energy by performing work.The transfer of energy take place in stepwise manner, at every step some amount of energy is used to carry out the work and this part of work constitute some coupled uphill chemical reactions in the biological systems like the biosynthesis of the molecules. The flow of the high energy electrons is a downhill process but on the contrary it provides energy for many uphill biochemical processes.
You might have heard that sun is an ultimate source of energy for most of the living beings on the earth. Have you given a thought what exactly it means? This process take place in Chloroplasts of the plants. Chloroplasts absorb the energy coming from the sun in the form of electromagnetic photons and this leads to the excitation of the electrons from the water molecule. At this point the journey of electron for stepwise energy distribution starts. Consequently the high energy electron is used to reduce the CO2 absorbed by plant leaves from atmosphere into Glucose, the food for most of the living being. And this magical process is termed as Photosynthesis.
All the mentioned transfer of electrons are always from one molecule to the other. For this transfer the electron donor, the one which gets oxidised and electron acceptors, the one which gets reduced are required. NADH and NADPH are the most important electron donors in the energy metabolism process in all animals and plant and many microbes. Glucose containing high energy electrons is oxidised in stepwise manner and, ATP and NADH are produced.
Here the high energy electron is used for the synthesis of ATP (and uphill process) while the electron is being transferred to next electron bearing molecule, NADH. ATP and NADH are required for the synthesis of other biological molecules. Also NADH can donates electron to the electron transport chain for further oxidation and release of the energy.
Eventually the electron is transferred to the Oxygen reducing it to the H2O i.e. where it came from. Complete oxidation of glucose forms highly oxidised CO2, which is then released in the environment which is then reabsorbed by the plants for the reduction. This is how the energy cycle repeats. We all survive on the energy provided from the food produced by plants with the energy provided by sun. Thats why sun is an ultimate source of the energy for all of us. So we are walking, speaking and doing all our stuff because the plants are fixing the energy coming from the sun. Thanks, again to plants!
Read more about electron transport.
Effect of electron environment on the pKa values of the amino acids
Electronegativity of multiple gropus in proteins compete for the electron. Chemical groups with electronegative elements like N and O, tend to strongly pull and withdraw the electron from neighbouring elements. This significantly influence the pKa values values of the amino acids.
The average pKa value for a carboxyl group attached to otherwise is unsubstituted aliphatic hydrocarbon is around 4.76, which is also the pKa value of the acetic acid. Whereas the pKa value of the carboxyl group of the amino acid Glycine is 2.34 which is more than 100 times acidic than the carboxyl group of the acetic acid.
Let us examine the structure of the Glycine molecule,
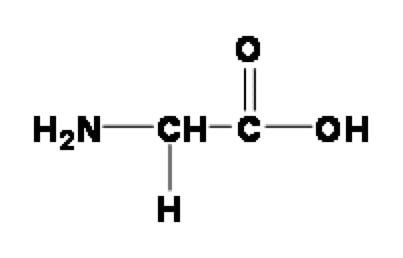
The alpha carbon of Glycine is attached to amino and carboxyl group. Amino group contains N as electronegative element pulling the electron from the carboxyl group, on the other hand oxygen atoms from the carboxyl group pull the electron from the amino group. Pulling of of electron by amino group forces the carboxyl group to loose the proton earlier in the acid base titration leading the reduced pKa of the carboxyl group. Same way, the pulling of electron by carboxyl group leads to lower pKa value amino group of Glycine than the average pKa values of the amino groups of other amino acids.
Electronic arrangement and biological pigments:
Many biological pigments are composed of long carbon chain of alternate single and double bonds called as dienes. This structural arrangement allows the delocalisation of the electrons because of which these molecules can be excited by low energy electromagnetic waves. This gives them colours, which are visible to human and other animals. Because of such compounds only, we see certain flowers coloured and birds with different coloured feathers.
This is how the a small sub-atomic particle can influence the crucial biological events. And it is possible because it can absorb the energy from the source, carry to different molecules and release the energy wherever it is required.


