The article “Activation energy (Ea) of reactions’’ briefly elaborated catalysis and influence of activation energy on the rate of reactions. We know that complete oxidation of glucose to H2O and CO2 can yield up to 36 ATP molecules.
Human brain needs up to 6 mmole (6X 10^20 molecular entities) of ATP molecules per minute. Preparatory phase of glycolysis produce three two carbon molecules which optimally give off the energy for the ATP production. In that phase, spontaneous conversion of glucose-6-phosphate to fructose-6-phosphate will take enormous time to meet the physiological demand of fructose-6-phosphate and will create a fructose-6-phosphate deficient environment and it won’t be sufficient to meet the actual need of ATP consumption by the body.
That is how the existence of the catalysts is more relevant; primarily to all the living entities and many enzyme technology based industries.

How catalysis work?
Every one of us have enjoyed the thrilling experiences during treks to the mountains, at least once in a life. We experience how tough is to climb the steep and elevated mountain and get passed it. Earlier when there were no road infrastructures, our ancestors used to climb the mountains on daily basis because there was no any alternative. Now with advanced machines, we have dug tunnels through the mountain which let us cross the mountain hassle-freely. Besides being easy what it has saved is a lot of time. This tunnel catalyses our climb over to the mountain.
Thus we need to supply a lot of external energy, many of the times as heat to increase the kinetic energy and to overcome the barrier of activation energy (Ea) in an uncatalysed chemical reaction. Biological catalysts reduce down the required activation energy of almost all the biological reactions.
Enzymes or any other catalysts can only reduce the amount of activation energy and they can not eliminate the need of the activation energy as we discussed in the article Why Learn Chemical Reactions?-1 most molecules lose maximum amount energy to attain the stability because of which even after reducing the activation energy some amount of energy is still needed. Then how do the enzymes manage to carry out such reactions?
There are chemical and biological catalysts. Enzymes are biologically most important catalysis agent. They are extraordinarily better than synthetic or inorganic catalysts in terms of their specificity and the environment in which they can function. According to some scientists catalysis is second most crucial aspect of life after the ability of the cells to reproduce and produce the replica.
As seen in the article, ‘Glucose oxidation: unpacking of the energy’ we saw that highly reduced sucrose or glucose synthesised in photosynthesis contains huge amount of energy. Thermodynamics does not favour the existence of large amount of energy stored in the limited space. Thermodynamically energy should be randomised and distributed to the surrounding. This makes the decomposition of the sucrose reaction thermodynamically highly favourable.
However when sugar is kept undisturbed, it can take years to release all the energy contained in it and convert to H2O and CO2. The reason is that demand of activation energy can’t be met at the necessary rate. In the living cells, enzymes can extract all that energy within minutes and produce H2O and CO2. The abilities of the enzymes can really be appreciated when they perform polymerisation of thousands of molecular building blocks within minutes. The process of biopolymerisation is thermodynamically highly unfavourable but with the help of enzymes all the living beings including viruses are able carry them out.
Now, It is clear that, enzymes speed up the reaction by lowering the activation energy. The graph of energy change with the progression of the reaction shows significant reduction in activation energy in the enzyme CATALYSED reaction.
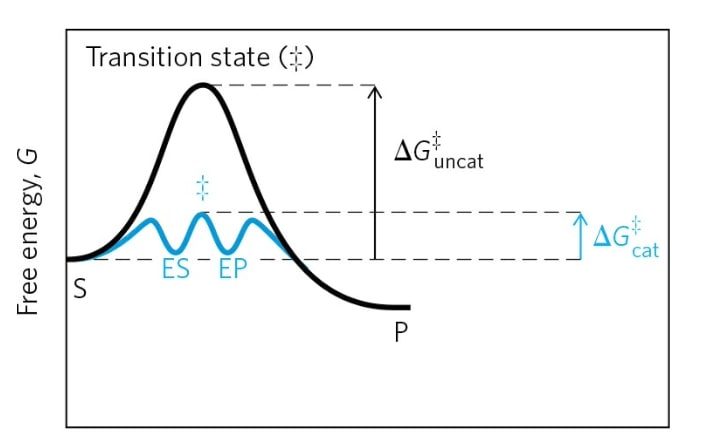
Mechanism of catalysis
It is also interesting to know how enzymes can do so? There are two major aspects of the enzyme catalysis. Most of the biomolecules have their defining chemical functional groups which are reactive. Different functional groups of the substrate molecules react with various functional groups of the amino acids side chain at the active site of the enzyme. Various weak interactions like hydrophobic interaction, ionic interaction and hydrogen bonding, release enough binding energy which help to stabilise the enzyme substrate complex, specify and catalyse the reaction.
Enzymes bind specifically to the functional groups present on the substrate and temporarily establish covalent bonds and other weak interaction with the substrate releasing energy thereby contributing to the activation energy of the reaction.
Many transient reaction intermediates form between the enzyme and substrate during the reaction resulting into an alternate low energy path for the same reaction. At active site the microenvironment of the reaction is very dynamic and very much different than the solution where the substrate molecules get activated so that they can be easily converted to products.
Catalysis is a magical phenomenon our biological world is blessed with. Without catalysis there is rarest possibility that a life could exist. Probably this can be one of the best reason to be always grateful about the life.

