Before reading further please find out what the following terms means.
Electronegativity, Dipole.
Structure of atom and electron orbitals
Earlier the atomic structure was shown like the atom consist of the nucleus which remains at the centre and the electrons revolve in the circular path around the nucleus. However it is very difficult to talk about their path of revolution around the nucleus. They do not exactly revolve around the nucleus in a circular path rather it is uncertain. Thus it is important to understand that electrons revolving around the nucleus are found anywhere but within the defined regions and only valence electrons, Lon pair of electrons and electron clouds act differently in chemistry.
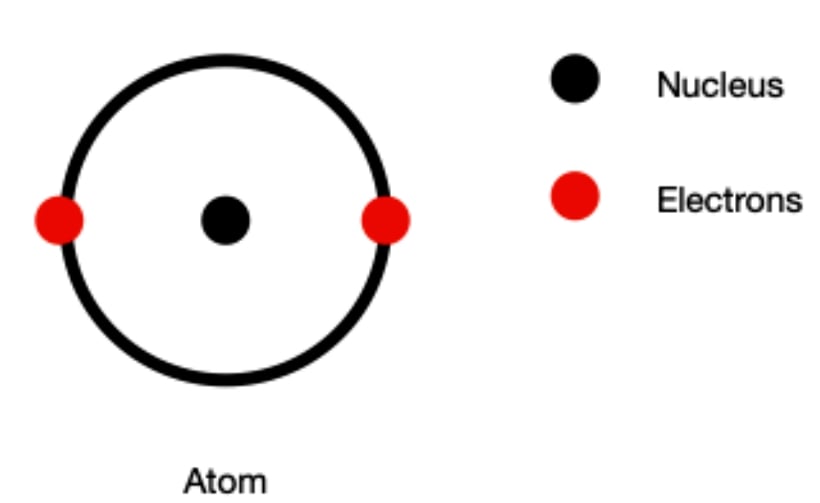
Depending upon the amount of energy it has, electron revolves around the nucleus at specific regions only. Such regions are termed as orbitals and each orbital has its unique energy levels. We have s, p d and and f energy orbitals with s and p being common to biological reactions. Each orbital has different shape around the nucleus which defines the the probable region of the occurrence of the electron and they can be found anywhere at any given time within this region. (read more about orbital)
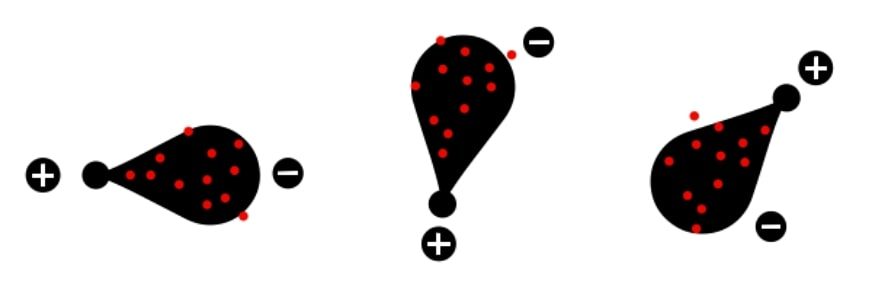
Random movement of the electrons and formation of transient opposite electric dipole
Influence of electrons on the molecular interactions
Apart from energy levels, the motion of the electrons is also influenced by the nearby atoms and their nuclei. Nuclei of the electronegative elements tend to attract the electrons from the nearby atomic orbitals. In simple manner the electronegativity of an element is an ability to pull the electrons from the nearby atomic orbitals causing redistribution of the total charge in the atom. For example, in case of water molecule Oxygen an electronegative element is combined with two Hydrogen elements.
Being electronegative, Oxygen atomic nuclei tend to attract the electrons from the orbital of hydrogen atom, thereby gaining partial negative charge which on the other hand at Hydrogen element induces partial positive charge, represented by δ-andδ+ respectively. And because of the attraction between opposite and partial positive and negative charges all the water molecules end up sticking together leading to high heat of vaporisation.
These type of sharing of electron pair between the nuclei of the electronegative element and hydrogen atom is termed as Hydrogen bond. Two strands of DNA are held together by such hydrogen bonds only. Also proper folding of the protein molecules and interaction between the enzyme and substrate is stabilised by hydrogen bonds and other interactive forces.
Let us look at some other biologically important molecules containing electronegative atoms. We have O2, N2 and CO2 as some of the smaller molecules containing electronegative elements. Being electronegative they have the tendency of pulling the electrons from neighbouring atoms and inducing local partial positive and negative charges. But this not the case with these molecules, in N2 and O2 electrons are shared equally by both the atoms making them unable to form hydrogen bonds with any other molecule.
On the other side there is big gap in the electronegativity of the C and O, thus C=O bond becomes polar because of transient, electric dipole . But again the structure of the CO2 molecule is O=C=O. Looking at the structure we can see that these Oxygen atoms are oppositely located thereby cancelling each other’s influence on the electrons of the Carbon atom. That is why such molecules have very poor solubility in water and are called as non-polar molecules.
Apart from the electronegativity the uncertain movement of the electron clouds of the electrically neutral atom leads to the formation of partial local charges within molecule. And generation of charge in molecule leads to the induction of opposite charge in nearby molecule. This brings these two molecules together with the forces of attraction called as van der waals interactions. These van der Waals forces are individually very weak forces but together they have significant impact on the structures of biological macromolecules like proteins, nucleic acids, polysaccharides and plasma membranes.
In the next article we will see other biologically important phenomenons that have primary involvement of the electron. Read about why to learn about chemical reactions?